Chemical laboratories often come into contact with all kinds of dangerous chemicals and perform some dangerous chemical operations. Among them, the hands of laboratory personnel are the most exposed parts of the hazard source. If there is a safety accident on the hands during the experiment, it will be The daily life and work caused great inconvenience.
Chemical protective gloves provide important protection for this, and can prevent hands from being injured by hazardous chemicals and ensure the safety of laboratory personnel.
Classification of chemical protective gloves
In the laboratory, the hazards of common chemicals to the hands are divided into three categories.
One is irritating or corrosive chemicals, which can cause damage to hands through direct or indirect contact. For example, the strong acids and alkalis frequently contacted in the laboratory can corrode the skin and cause severe pain; the hydrofluoric acid mist can irritate the hands and cause dermatitis and other symptoms.
The second is burns to the hands after high-temperature burns. For example, magnesia calcined at a high temperature is prone to burn hands when taken out.
The third is frostbite to the hands of low-temperature chemicals. For example, liquid nitrogen, dry ice, etc. in the laboratory, improper protection during operation can easily frostbite the hands.
In chemical laboratories, commonly used chemical protective gloves are mainly divided into the following categories:
Natural rubber gloves (latex gloves)
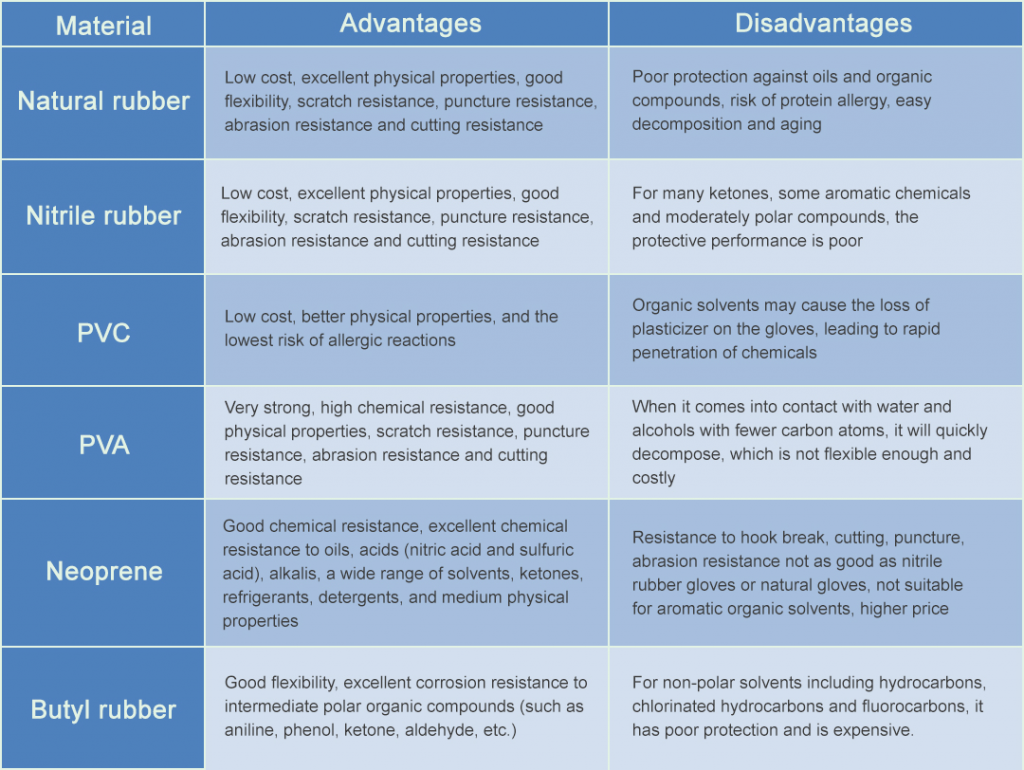
The material is generally natural rubber collected from rubber trees, and is made of elastic solids after coagulation and drying processes. The main component is polyisoprene.
This type of glove has good chemical resistance to general chemicals, and is suitable for common low-concentration acids, alkalis, aldehydes and ketones, and can be continuously operated in low-concentration acid and alkali solutions. Good wear resistance and puncture performance.
Nitrile rubber gloves
The main material is composed of acrylonitrile, butadiene and rubber. Nitrile rubber gloves generally have good chemical resistance and mechanical strength, wear resistance and puncture resistance. At the same time, because it does not contain protein allergens, it has the lowest allergic irritation to human skin. It is suitable for the protection of conventional acids and alkalis. It has good protection against grease, toxic and corrosive substances. It is also the most widely used chemical protective glove in the laboratory.
Polyvinyl chloride (PVC) gloves
The main materials are PVC paste resin, plasticizer, viscosity reducer, heat stabilizer, etc. PVC gloves have strong chemical resistance and have a certain degree of protection against chemicals in the laboratory. Abrasion resistance is good, but exposure to organic solvents will accelerate the loss of plasticizer in PCV gloves, resulting in a decrease in chemical protection performance, which has certain limitations.
Polyvinyl alcohol (PVA) gloves
The main material is polyvinyl alcohol. Its resistance to dangerous organic solvents is particularly outstanding, and it has a good protective effect on most organic solvents. Good physical properties, puncture resistance and abrasion resistance, but more cumbersome to wear.
Neoprene gloves
The main material is produced by chloroprene emulsion polymerization. It has high oxidation resistance, resistance to hydrocarbon solvent swelling, good chemical resistance, physical strength and aging resistance. However, the price is relatively expensive due to its raw materials and process procedures.
Butyl rubber gloves
The main material is synthesized from isobutylene and a small amount of isoprene. It has super durability to strong acids, strong bases, toluene, etc., and has high physical and chemical resistance. It is suitable for the operation and use of glove boxes, incubators and other related equipment in the laboratory.
Performance of chemical protective gloves
The Performance of Chemical Protective Gloves is Mainly Evaluated from Three Aspects.
One is permeability: protective gloves undergo a process of intermolecular permeation during contact with chemicals. First, the surface of the protective glove absorbs the molecules of the chemical substance in contact. According to the principle of concentration diffusion, then the molecules of the chemical substance diffuse in the glove material, and finally in the other part of the glove. Release on one side.
The second is penetration: when there are large pores and gaps on the protective gloves, chemical substances penetrate in non-molecular form, which greatly increases the risk of harmful substances contacting the skin.
The third is degradability: after chemical substances come into contact with protective gloves, the relevant physical properties of the glove material will be attenuated, such as swelling, stretching, and rupture.
In actual use, the protective performance of gloves is closely related to many aspects. The main factors are frequency of use, method of use, and the influence of chemical reagents.
The use of protective gloves is divided into disposable and repeatable. In the process of use, if the frequency of use is too high, physical degradation such as tearing and swelling is likely to occur, resulting in a decrease in chemical resistance. When used improperly, it can also cause damage to protective gloves. If long-term exposure to certain organic solvents (such as alcohols, halogenated hydrocarbons, ketones, aliphatics, etc.), they will affect the permeability of the gloves, thereby weakening the protective performance.
Selection requirements for chemical protective gloves
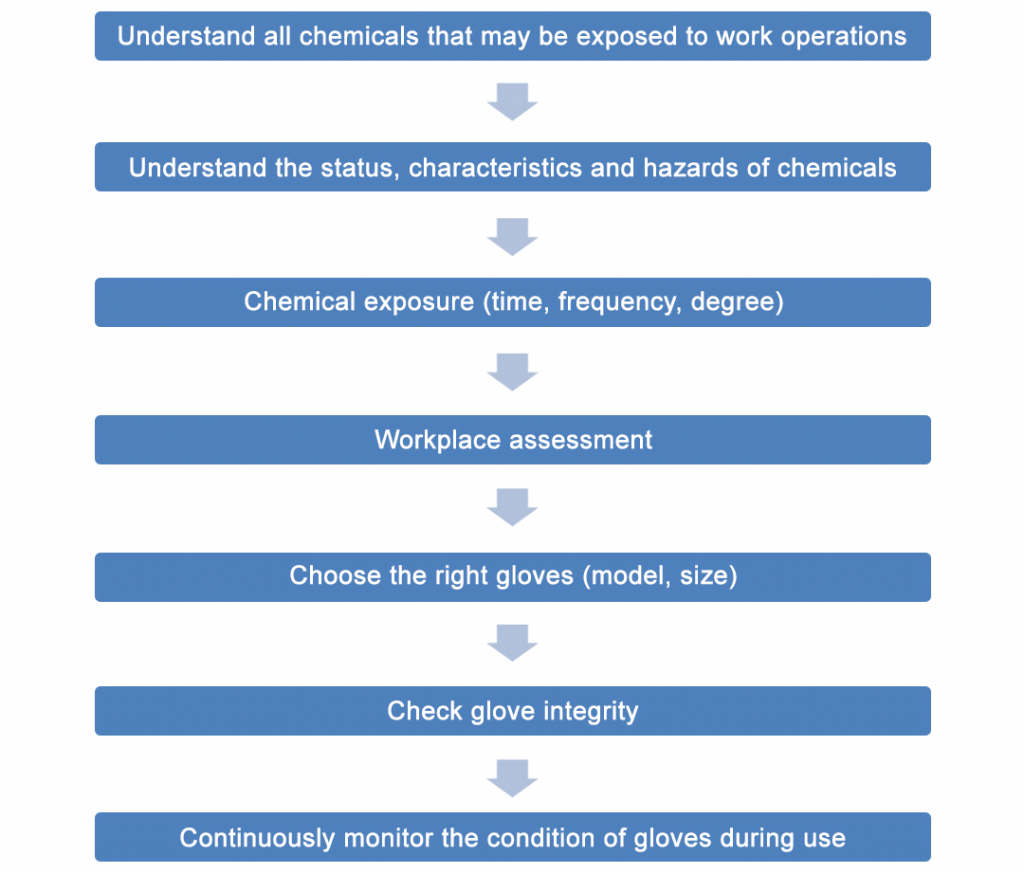
There are many types of chemical protective gloves in the laboratory, and each has a certain protective effect on certain chemical substances. Therefore, you can refer to the process in Figure 1 to select suitable protective gloves. The advantages and disadvantages of various chemical protective gloves are shown in Table 1.
Choosing the right protective gloves is only the first step in protective work, and laboratory personnel must also further understand the precautions during use.
To check whether the gloves have small holes or damaged or abraded places, especially finger joints, the gloves can be inflated to 1.2 to 1.5 times of the original value through the inflation method, and then immersed in water to check for air leakage.
Wash your hands before wearing gloves to prevent bacteria carried on your hands from breeding in a warm and humid environment after wearing gloves, causing dermatitis. If there is a wound on your hand, heal or cover the wound before wearing gloves to prevent bacteria and chemicals from entering the body and causing secondary injury.
When using gloves, pay special attention not to contaminate the skin and clothes with harmful substances to prevent secondary pollution. Never share gloves to prevent cross-infection.
Gloves contaminated with hazardous chemicals should be treated as chemical waste, and protective gloves containing biological contaminants should be treated as biologically hazardous materials, and they should not be discarded at will.
After taking off the gloves, wash your hands and rub some hand cream to replenish natural protective oils and prevent dry hands. Don’t ignore any skin diseases such as erythema, itching, dermatitis, etc. If your hands are dry, itchy, or air bubbles, please consult a doctor in time.
When contacting various chemicals in the laboratory, consider the hazards of the chemicals and select the highest level of protection according to the characteristics of the glove material. You can also consider wearing double-layer (different material) gloves for protection. In addition to paying attention to the material and type, it is also necessary to pay attention to the size. In short, there is no universal protective gloves. It is the best policy to choose the right and appropriate gloves according to the chemicals in contact.