With the increasing energy demand from mobile devices, electric vehicles and large-scale energy storage systems, it has become particularly important to develop batteries with higher energy density, longer cycle life and higher safety performance. Lithium-metal batteries have become a research hotspot due to their theoretical energy density of more than 500 Wh kg-1. However, the commercialization of Li-Metal batteries is limited by their limited cycle life, which is mainly due to the poor stability of the electrolyte-electrode interface. Conventional electrolytes are difficult to be compatible with lithium-metal negative electrodes and high-voltage positive electrodes, and often require a compromise between the two. Therefore, designing electrolytes with highly stable interfaces at both the negative and positive electrodes is the key to realizing high energy density lithium metal batteries.
Recently, Xuefeng Wang, a Distinguished Researcher at the Institute of Physics, Chinese Academy of Sciences (IPS)/National Research Center for Condensed Matter Physics (NRCP) in Beijing, in collaboration with researchers from Peking University, the University of Science and Technology of China (USTC), and Soochow University, reported a contact ion-pair aggregation (CIPA) electrolyte with a unique nanoscale solvated structure. In this case, the ion pairs are densely arranged to form large-sized aggregates, which can promote the rapid interfacial reduction kinetics of lithium-metal anode through the collective electron transfer process, thus forming a stable interface. A 505.9 Wh kg-1 lithium-metal flexible pack battery assembled with a high-nickel cathode had an energy retention of 91% after 130 cycles.
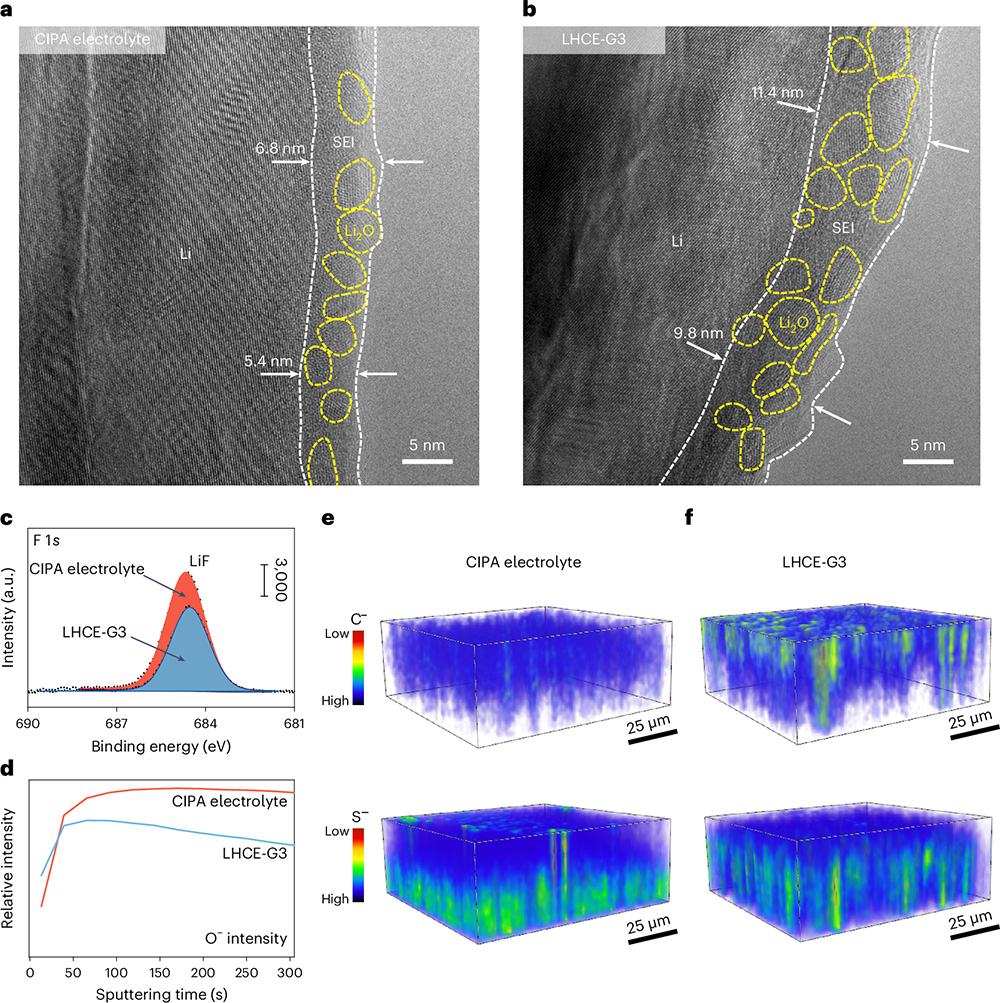
This work used cryo-transmission electron microscopy in conjunction with a series of advanced surface analysis techniques to investigate the structure, composition and spatial distribution of the solid electrolyte interfacial film. Structurally, the CIPA electrolyte forms a thin and uniform SEI film on the surface of the lithium metal anode. The average thickness of this film is about 6.2 nm, which is lower than that of the SEI film formed in the localized highly concentrated electrolyte. The SEI films formed in both electrolytes exhibited organic-inorganic composite structures, with the Li2O nanocrystalline grain sizes and distributions in the SEI films formed in the CIPA electrolyte exhibiting higher homogeneity. In terms of composition, the SEIs formed by LHCE-G3 electrolyte showed less LiF content and stronger C- and O- signals compared with CIPA electrolyte, indicating less FSI-anion decomposition and more severe solvent decomposition. In terms of spatial distribution, the C- and S-elements in the SEI membrane derived from CIPA electrolyte are more uniformly distributed from the inside to the outside, whereas the LHCE-G3 electrolyte detects obvious C- signals in the outer layer of the SEI membrane, whereas the S- signals are concentrated in the inner layer, suggesting that the reduction behavior of the LHCE-G3 electrolyte is inhomogeneous, which triggers localized lithium deposition and leads to lithium dendrites formation. A synthesis of the interfacial characterization results reveals that the FSI-anions in the CIPA electrolyte are rapidly reduced by a collective electron transfer mechanism to form a LiF- and Li2O-rich SEI film with low organic content. This stabilized SEI layer provides effective protection for the lithium metal anode and prevents further side reactions, thus enhancing the cycling stability and safety of lithium metal batteries.

Etelux News